
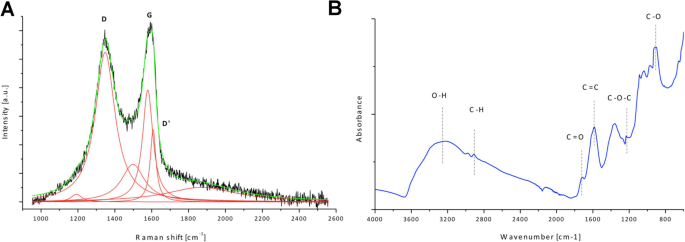
According to the Lerf-Klinowski model, the carbonyl and carboxylic groups remain attached to the edges of the sheet, while the hydroxyl and epoxy groups are found on the basal plane. Graphene oxide (GO) can be best described as a single layer planar hexagonal array of carbon atoms to which functional groups, including carboxylic acid, hydroxyl, epoxy, and carbonyl groups, are attached. Graphene oxide syntheses via chemical methods using acids and oxidants are the most widely followed procedures, which can be further reduced to produce graphene. In spite of the viable structure-property relationship, large volume production of graphene is still a challenge. Its different synthesis routes and the possession of unique mechanical, thermal, and electrical properties make it a material of great interest. Graphene is a flat 2D layer of carbon atoms packed in a honeycomb lattice and is the basal building block in all graphitic materials. Such shift can be ascribed to restoring of electronic conjugation of the C=C bonds in CCG.


The UV-Vis spectra showed absorption peak at 230 nm for GO colloids that shifted to 260 nm for the CCG colloid. Both of the colloids exhibited average size of ~1 micron in the low pH range, whereas for higher pH the size ranged between 300 and 500 nm. Poor stability of the colloids was observed for both GO and CCG colloids at both extremes of the pH scale. The GO colloid was stable in the pH range of 4–11, whereas the CCG colloid gained stability at a relatively narrower pH range of 7–10. The effect of pH on particle size, particle charge, and light absorption of the aqueous colloids of GO and CCG was studied with titration against HCl or NaOH, to find the ideal characteristics for a stable dispersion. Graphene oxide (GO) was prepared by modified Hummer’s method, and chemically converted graphene (CCG) was prepared by further reduction of the aqueous GO colloid.


 0 kommentar(er)
0 kommentar(er)
